Jacob's Ladder
If you have ever seen any of the old, B-grade science fiction movies,
you've probably noticed a Jacob's Ladder zapping away in a corner of the
mad scientist's laboratory. Although of no great practical use, the
Jacob's Ladder provides an engaging display which illustrates many interesting
physical phenomena. The device is fairly simple, consisting of two
vertical electrodes which diverge slowly from bottom to top and a high
voltage, modest current, power source. The lower ends of the electrodes
are placed close enough that the power supply voltage can cause dielectric
break down of the intervening air, establishing a conductive plasma channel,
or power arc. The arc is much hotter (~5000C, as hot as the sun's
photosphere), and therefore less dense, than the surrounding air and will
rise up the electrodes and lengthen until the available voltage and current
can no longer provide enough power to maintain the air in a plasma state.
The arc then quenches and reforms at the lower end of the electrodes and
the cycle repeats.

The picture above is of my first, rather crude, Jacob's Ladder.
The picture was taken with no flash, in order to force a longer exposure
time from my Polaroid camera. The electrodes are two artfully bent
lengths of 1/4" OD copper tubing bolted directly to the terminals of a
120 mA, 15KV neon sign transformer. Although the picture is a bit
blurry (due to a combination of the long exposure time, hand held camera
and shifting illumination) you can see on my variable transformer that
the needle of the upper meter (0-15 amps) is pegged and the lower meter
(0-150 volts) is at ~ 80% (120 volts), providing ~1800 watts to the neon
sign transformer. Although it was easy to build, this Jacob's Ladder
was inconvenient to transport, set up and somewhat fussy to operate (the
soft copper electrodes always had to be rebent a little to get it to work
well). Below is an improved version.
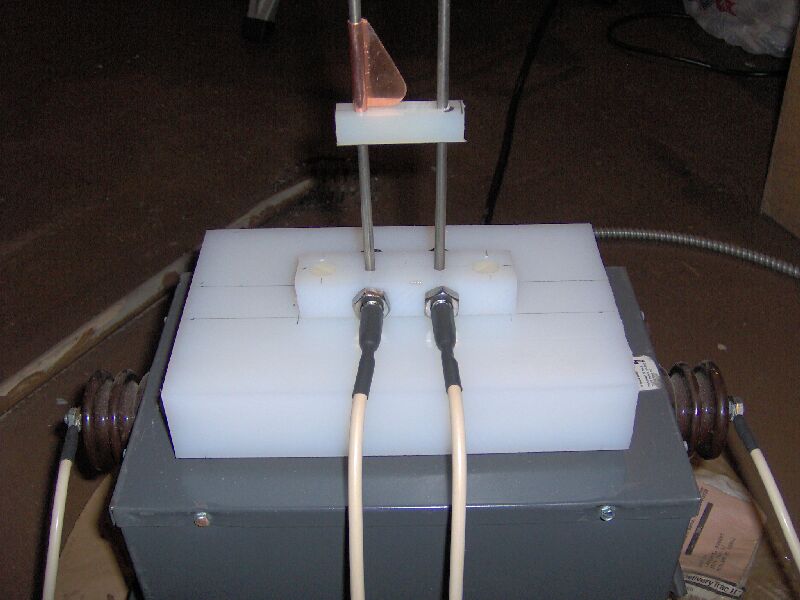
This is a picture of the base of my latest effort at a Jacob's Ladder,
sitting on top of and plugged into my NST. The vertical electrodes
are six foot lengths of 3/16" diameter 316 stainless steel rod. The
middle size plastic block is a 4" X 1" X 3/4" piece of ultra high molecular
weight polyethylene (UHMWPE) which was drilled and tapped to accept two
1.5" lengths of 3/8"-16 threaded 18-8 stainless steel rod, 1.25" apart.
The threaded rods were secured in their threaded holes with SS nuts and
a 5/32" hole drilled into one end of each rod to accept a banana plug.
The middle block was then bolted to the large, 5" X 8" X 1.5", UHMWPE block
with 1" long, 1/4"-20 nylon machine screws and two 3/16" holes drilled
through the middle block, threaded rods and large block to a total depth
of two inches. The bottom of the large block is also fitted with
four small rubber feet. The upper, smallest, plastic block is a 2.5
inch length of 1/2" square nylon, with two 3/16" holes drilled 1 5/16"
apart and slightly enlarged with a tapered reamer (so they are sort of
hour glass shaped in cross section, to prevent binding of the rods).
Sliding this block up or down the rods varies the angle at which the rods
diverge as they rise. In order to get the arc to form above this
point, a 1.5" long piece of copper sheet (0.021" thick, alloy 110) with
one edge rolled around the electrode and the other cut, filed and polished
to form a ramp, was added to one electrode. The parts were all from
McMaster-Carr
supply company, although everything but the electrodes were scrap from
other projects.

These pictures show the new Jacob's Ladder in operation. The
pictures were taken with an HP M305 digital camera, with the flash turned
off to force a long exposure time. In the left image, the electrodes
are (relatively) clean. In the middle image, the electrodes have
been swabbed with an aqueous sodium chloride solution (salt water).
In the right image, the electrodes have been swabbed with an aqueous calcium
chloride (de-icer) solution. As the arc travels along the electrode,
a portion of the salt is vaporized. Electronic excitation of the
liberated metal ions results in the characteristic photon emission (yellow
for sodium, orange for calcium). Other metals whose salts might be
interesting include: strontium (bright red); lithium (red); barium (green);
copper (blue).

I recently found a supplier of various metal carbonates, United
Nuclear, and converted them into the acetates by dissolving them in
vinegar. This composite picture shows the results. The strontium
and barium acetates produced rather faint emissions and the copper acetate
was rather disappointing (copper chloride is claimed to produce a true
blue color, while other copper salts typically produce greens). I
suspect that the acetate is decomposing to relatively non-volatile copper
oxide, producing a carbon emission, like a candle flame. If I can
find a supplier of hydrochloric acid, I may revisit this.
Home




